Categories for Abortion Pills
 July 28, 2016 9:14 am
Published by Marcella
July 28, 2016 9:14 am
Published by Marcella
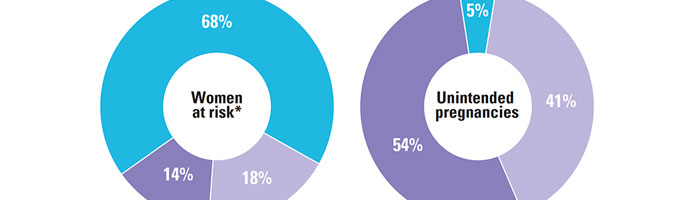
Unplanned pregnancies are a problem not only in the United States, but throughout the world. According to a research, 90 percent of abortions happen because of unintended pregnancy, and 50 percent of these occur in developing countries, where access to birth control lacks. In other cases, female do not understand the importance of using contraception and either avoid it or utilize these incorrectly, leading to a pregnancy they had not expected. Often people with unwanted pregnancy at a young age may be compelled to not pursue a full-term birth. At an early gestation, women can buy abortion pills or undergo surgical removal of fetus to cause end of pregnancy. Other options are parenting (bringing up the child), or adoption (giving up child in foster care). All these choices need to be well understood, before opting one that is best for the person and her wishes. Here are ways one can plan a pregnancy: Perform an unprotected intercourse in case trying to conceive. Utilise contraceptive in case there is no will to get pregnant then. Talk to partner about contraception use. Understand which birth control is suitable for use. Utilise the device, medicine etc, chosen correctly. Check for results after completed... View Article
 July 11, 2016 9:35 am
Published by Marcella
July 11, 2016 9:35 am
Published by Marcella
A medical pregnancy termination caused by abortion pills allow a female privacy of her home, and control over the procedure at her own convenience and time. The person can possibly have a trusted-someone be around during the regimen. The female may have to take some painkillers in handling cramping, but at least does not have to take anesthesia which is the case during a surgical process. Here is a list of other pros of abortion pills: More Private: It is a successful method, feels like natural ‘miscarriage’, and not at all invasive. The sooner it is done, better are the results, and less painful it is. Less Expensive: If cost of the process matters to you, then medication process is one of the best options in rival to surgical regimen, which can cost to $500 or more, while MTP Kit abortion pills may be available at just $200 or under. No Infections: The risk for infections is lower in medication technique, as there are no instruments intruding in the uterus thus there is no concern of sterilizing anything to end the pregnancy. Earlier Termination: You can choose to cease your pregnancy in as less as 4 weeks of gestation, or... View Article
 July 4, 2016 12:13 pm
Published by Marcella
July 4, 2016 12:13 pm
Published by Marcella
A pregnancy ending can be performed either via oral intake of abortion pills or a surgical termination regimen. A woman can choose either of the two in case she is in early pregnancy. Abortion pills combine Mifepristone and Misoprostol tablets for the removal of pregnancy parts from the body. It is a non-invasive method compared to surgical aspiration. In surgery-aided pregnancy termination, the doctor numbs the cervix and inserts an instrument to clean out the uterus of pregnancy particles. The medical procedure seems like a natural early miscarriage technique, against the invasive method. Also, in medication treatment, the female can be at home, while in the latter, hospital care is necessary, making surgery costlier than the drug regimen. Here is a brief comparison between the two methods, which will help you decide, which is the better way of ending pregnancy- is it the abortion pill or surgery? How Far Along Pregnancy Do They Work Medical abortion can be done up to 10 weeks of pregnancy. Success rates are low after 12 pregnancy weeks. Most of the pregnancies are terminated in less than 8 to 10 weeks, making abortion pills convenient for women. Surgical pregnancy termination can be done from the... View Article
 June 28, 2016 9:57 am
Published by Marcella
June 28, 2016 9:57 am
Published by Marcella
The do-it-yourself or DIY abortion is a home-based method to end pregnancy, done with medicines, and normally in complete privacy. The technique is picking up in the U.S and the world, especially in developing nations. The reason why people buy MTP Kit online could be influenced by either a regard for having the medications delivered at home, or inability to access a local healthcare. Be it whichever care, the medicines Mifepristone and Misoprostol are potent remedy to unwanted pregnancy, and terminated it within 10 to 16 days. It is a process, which has no space for surgical instruments and anesthesia. Medical technique is in vogue at least for those who really need to put an end to their pregnancy. However, it may not be as easy as it seems for all. What are the Hurdles to Medication Access? With political strife between healthcares and laws to regulate these, pregnancy ending has become one of the most talked about topics inviting both critiques and supporters. Some areas do not even allow females who are victims to abuse and forced activities leading to pregnancy- in terminating the conception. However, in most places one can buy MTP Kit without much of a problem.... View Article
 May 17, 2016 11:13 am
Published by Marcella
May 17, 2016 11:13 am
Published by Marcella
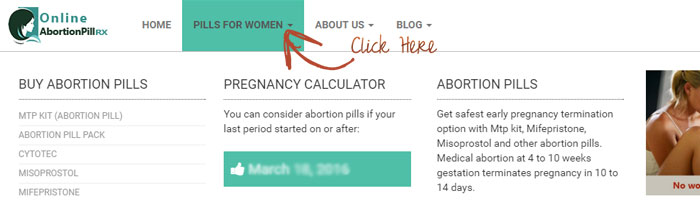
Pregnancy Calculator will help if you are considering abortion pills? Pregnancy Calculator is the easiest way to check how many days you are pregnant to get assure of termination with abortion pills. Check the date flashed on the pregnancy calculator of your last period. Usually upto 8 to 10 weeks are considered safe for termination with abortion pills. Contact us for more consultation. Click on “Pills For Women” on top menu, to get pregnancy calculator.
 April 28, 2016 12:04 pm
Published by Marcella
April 28, 2016 12:04 pm
Published by Marcella
Since decades, medication methods are used to cause abortion. A combination of tablets are taken to empty the uterus. The agents used to terminate a pregnancy are anti-progesterones and prostaglandins. In the United States, Mifepristone is marketed by the Danco Laboratories, LLC, which is under Mifeprex as trade name, sold to the physicians. The medication can soften and dilate cervix leading to decidual necrosis that is ‘placental detachment’. It increases prostaglandin releasing from uterine lining, and with Cytotec (brand name for Misoprostol), frequents uterine contractions and sensitivity to prostaglandin. Thus, myometrial contractions expel pregnancy tissues by ripening cervix. In few days the womb is left empty of all the pregnancy sections. Progress of Abortions Pills – Then and Now In 1980, a product named Mifepristone was developed, which were later marketing in the U.S as RU-486 and Mifeprex, used initially to begin pregnancy termination. The medicine blocks progesterone hormone in body, by binding to the hormone’s receptors. In 1985, Mifeprex was discovered to sensitize myometrium to prostaglandin. Thus administration of it with Cytotec shot efficacy of medical abortion from 80 to 95 percent. In 1988, Mifepristone gained licensed use in France, which was given along with Misoprostol (prostaglandin analogue that... View Article
 April 13, 2016 10:48 am
Published by Marcella
April 13, 2016 10:48 am
Published by Marcella
1. What is Mifeprex Abortion Pill, and how does it Work? Mifeprex is a drug that blocks hormone (progesterone) needed for continuing pregnancy. The medicine is also known by its generic name, Mifepristone. When the medicine is used with another drug Misoprostol, it can cause an end to early pregnancy (4 to 10 week gestation, or up till 70 days from the first day of female’s last menstrual period). 2. Who must not take Mifeprex? Some females should not use Mifeprex. A person must not take this drug, if she is more than 70 days due her pregnancy, or if she: On long-term corticosteroid therapy, ectopic pregnant (pregnancy outside uterus). Is allergic to Mifepristone, Misoprostol or similar medications. Has adrenal glands problem or inherited porphyria. Has IUD (intrauterine device) inserted (must be removed before intake of Mifeprex). Is taking anticoagulant drugs or products, or has bleeding problems. 3. What are the approved changes done to Mifeprex Label by the FDA on March 29, 2016? Mifeprex was first approved in 2000 by the FDA. In 2016, a supplemental application of the medicine given by its marketing company was approved. The approval stated few changes in the dosing of Mifeprex and dosage... View Article
 July 28, 2016 9:14 am
Leave your thoughts
July 28, 2016 9:14 am
Leave your thoughts
















